Exploring the power of
Bacteriophages
Proteon utilizes the unique capabilities of its bacteriophage products to establish an environment conducive to the growth of beneficial microflora. As we confront challenges posed by antimicrobial resistance (AMR), bacteriophages emerge as a promising and eco-friendly tool for combatting bacterial infections while preserving microbial balance.
Definition
Bacteriophages
They are commonly known as phages, represent the smallest, oldest, and most abundant naturally occurring organisms on Earth. Bacteriophages, are specialized viruses with the ability to selectively target and neutralize specific bacterial pathogens. These viral entities play a crucial role in maintaining microbial balance. Bacteriophages are good viruses that infect only their specific host bacteria. By preventing the overgrowth of specific pathogenic bacterial strains, they allow other beneficial bacteria to thrive, contributing to a more balanced and diverse gut microbiota.
Characteristics
Key Features
Proteon utilizes the unique capabilities of its bacteriophage products to influence the gut microbiota, establishing an environment conducive to the growth of beneficial microflora. This precise strategy plays a key role in preventing dysbiosis, enhancing overall gut health, and bolstering immune functions.

Highly targeted impact on bacteria
Abundant and diverse in nature
Support microbiome balance
Safe, natural, non-toxic & non-GMO
Do not contribute to antibiotic resistance
100% biodegradable organisms
Leave no residues in the body
Effective against even multidrug-resistant strains
More about
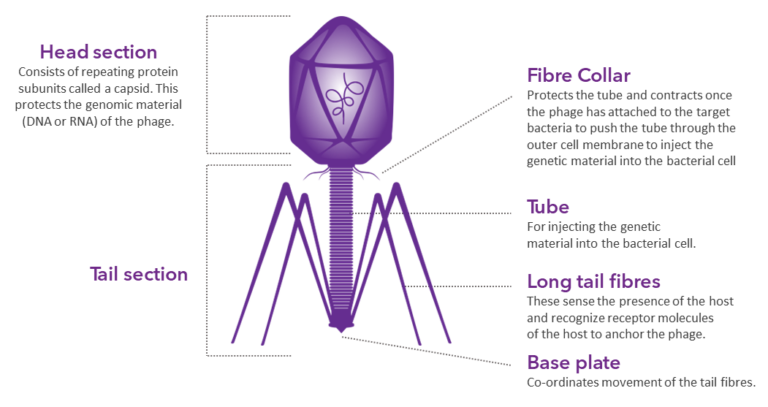
Bacteriophage Structure
Bacteriophage structures are diverse, but most of them share some common characteristics. The most distinctive example is T4-like phage structure composed from head and contractile tail section.
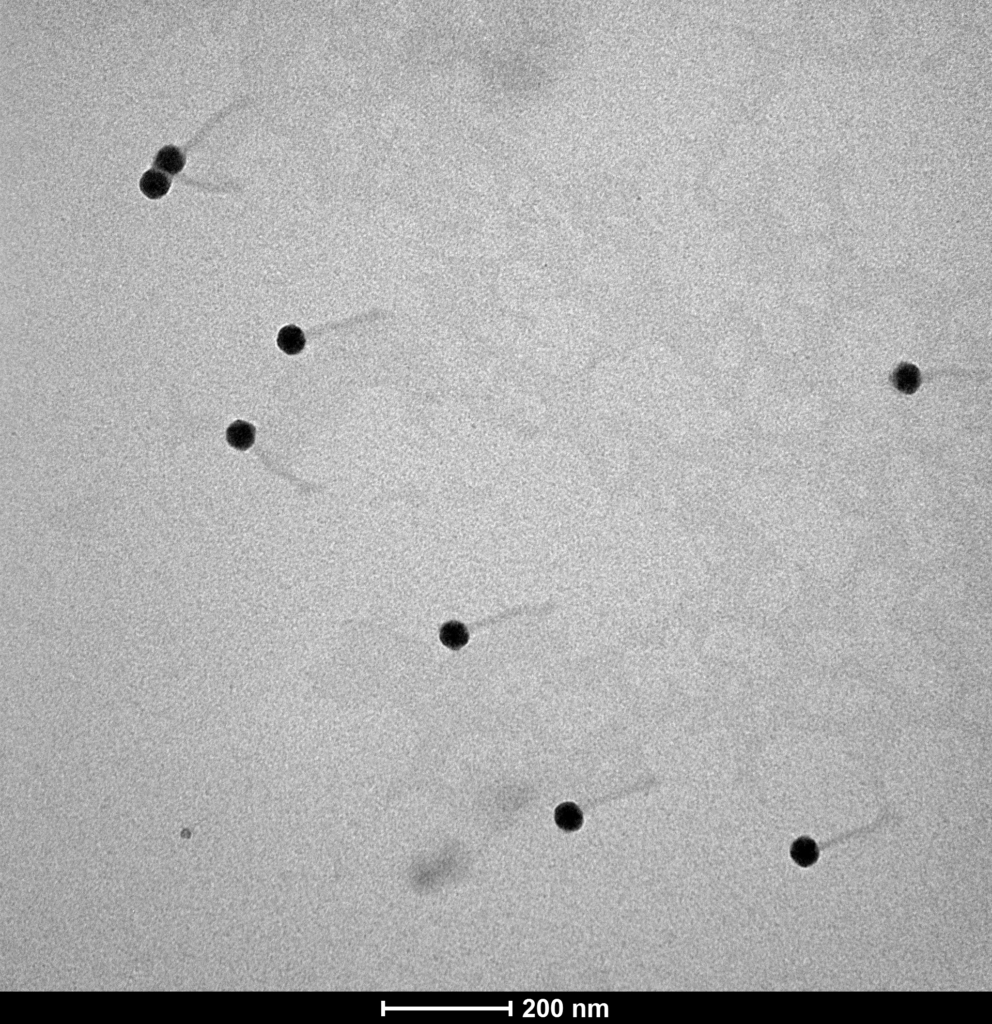
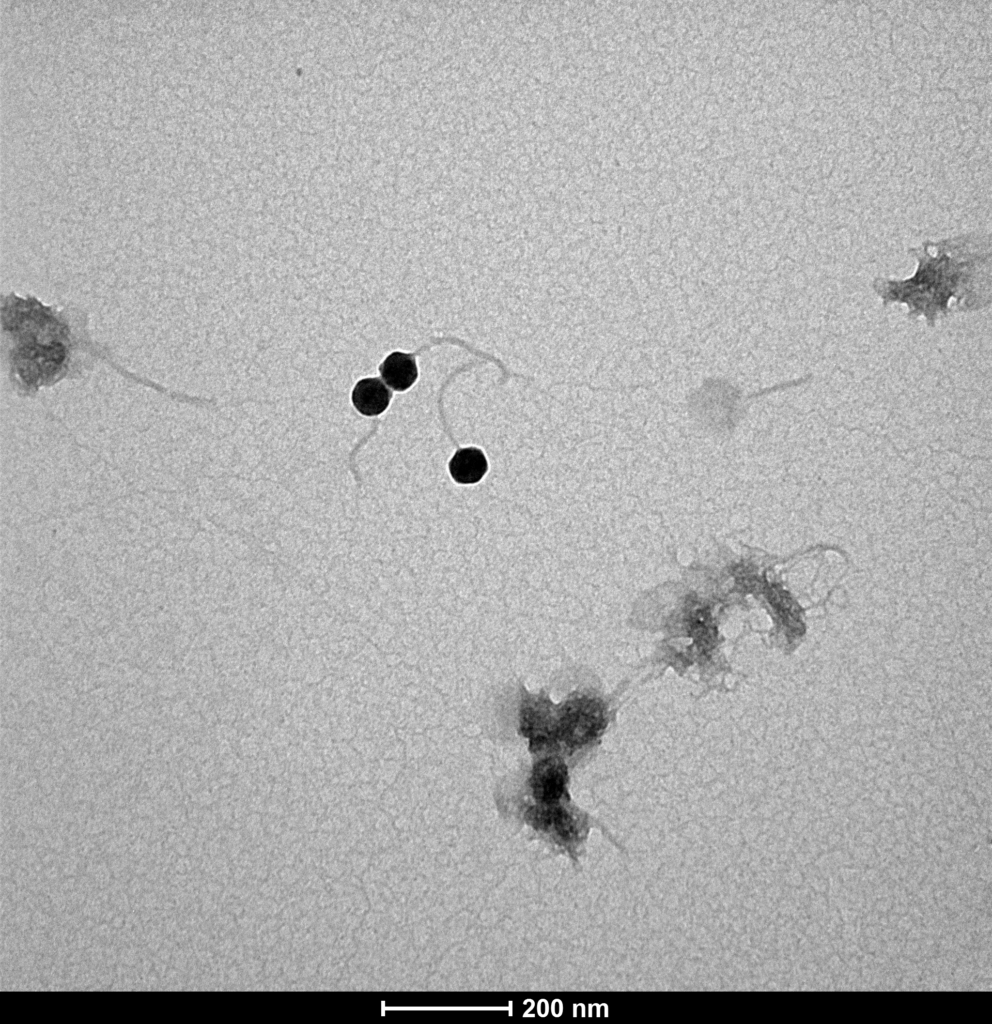



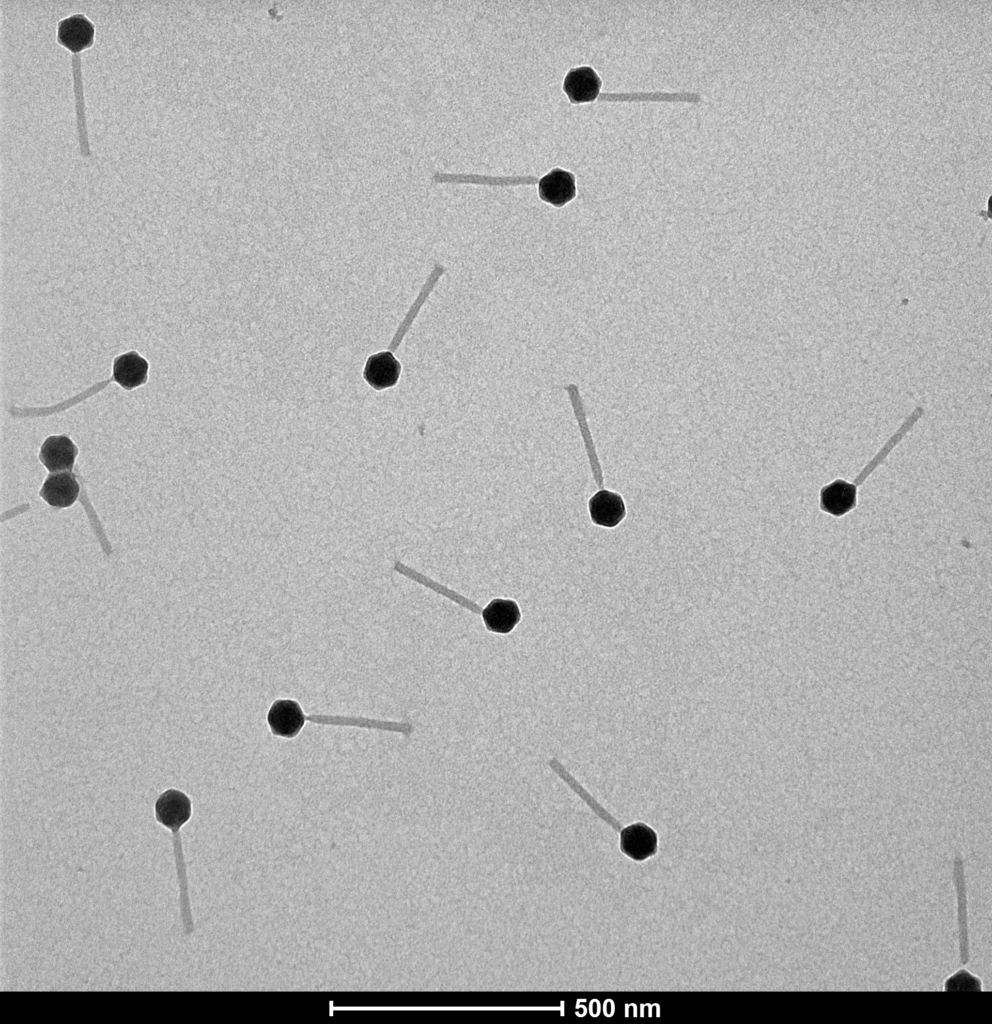
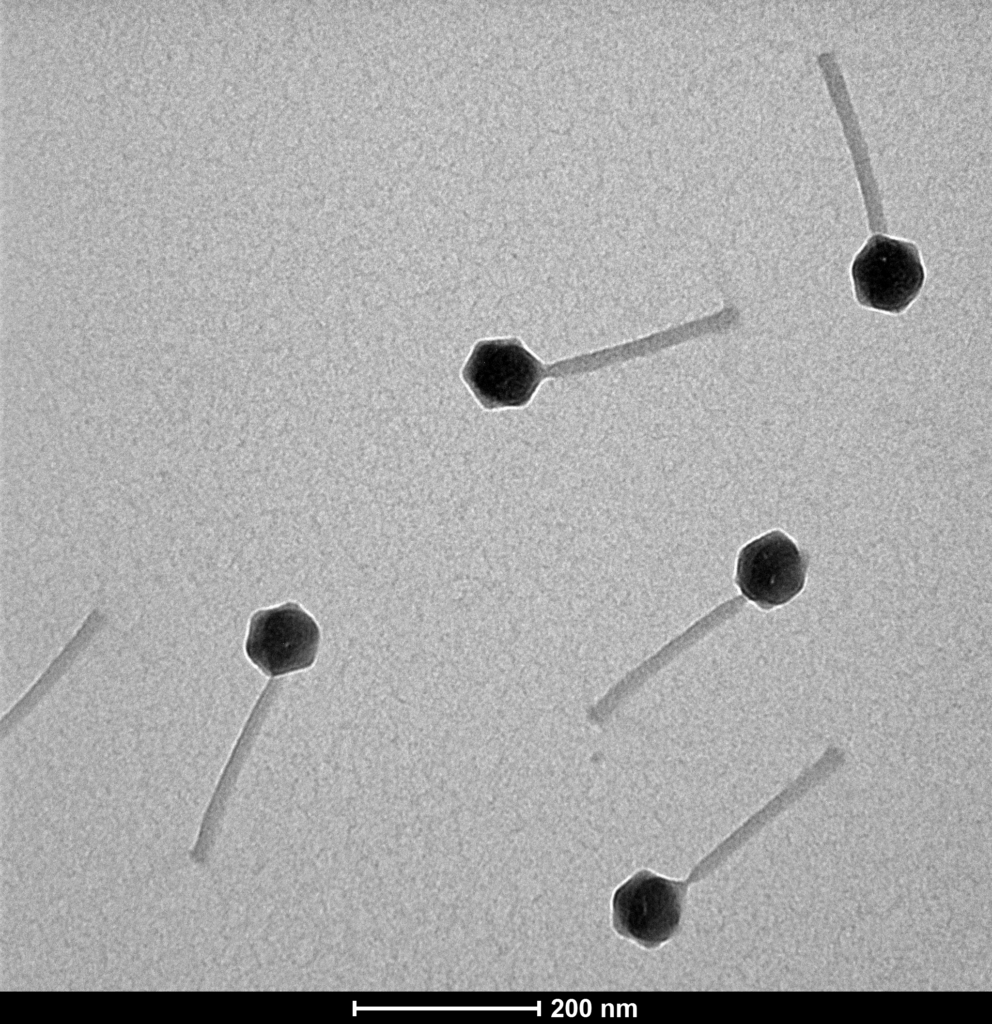
Proteon Photo Resources – Examples of Bacteriophages under Microscope
Mechanism of Action
Bacteriophage Host Cell Attachment Mechanism
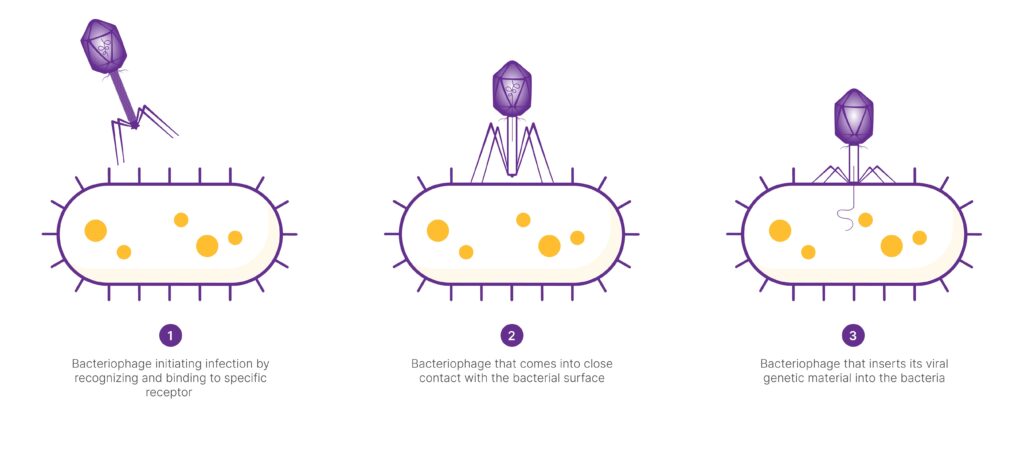
Bacteriophages initiate infection by recognizing and binding to specific receptors on the bacterial cell surface using tail fibers or tail spikes. This interaction facilitates the close contact needed the insertion of viral genetic material into the bacterium.
Upon infecting a bacteria, a phage can either immediately produce new phage particles and lyse (break open) the bacterial cell, or it can integrate its genetic material into the bacterial genome, lying dormant until triggered to replicate. Due to their ability to kill bacteria, phages are being explored as natural antimicrobials, alternatives to antibiotics.
Classification
Variations in Mechanisms of Action
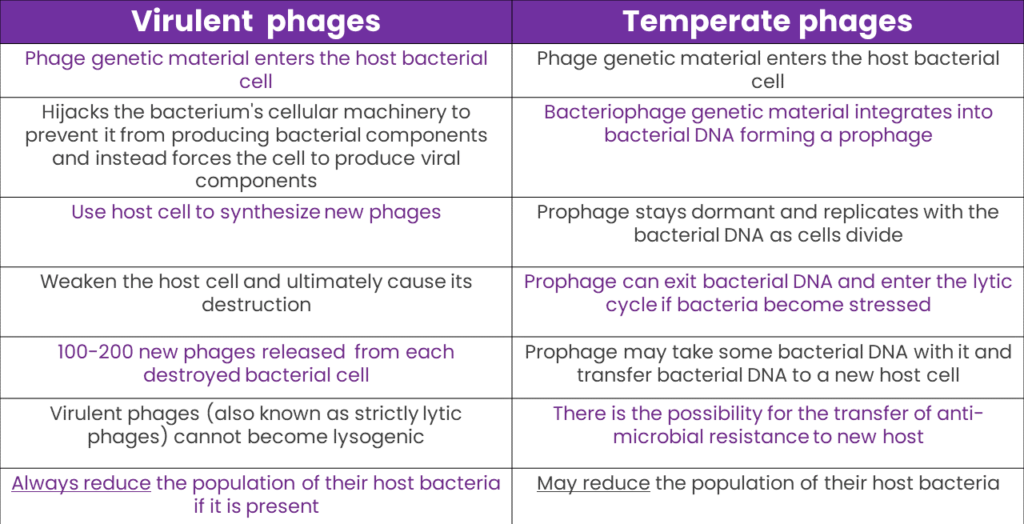
Bacteriophages are broadly classified into two primary classes: virulent (lytic) and temperate (lysogenic). Both classes recognize and attach to specific bacterial hosts and inject their genetic material into them. However, their subsequent actions diverge. Virulent phages replicate rapidly within the host bacterium and eventually cause its destruction, or lysis, releasing new phage particles to infect other bacteria. In contrast, temperate phages integrate their genetic material into the bacterial DNA, lying dormant and coexisting with the host. It’s only under certain conditions that they are triggered to activate and adopt a lytic lifestyle. These fundamental differences in life cycles dictate their interactions with bacterial hosts and their potential applications in fields like medicine.
At Proteon, we utilize virulent phages for our bacteriophage solutions
Application
Significance of Bacteriophage Selection in Animal Production
A comparison between virulent and temperate phages, highlighting their similarities and key differences, underscores the importance of careful bacteriophage selection for animal production. Understanding their distinct mechanisms can inform strategies for enhancing animal health.
Overview
The Challenges behind Natural Bacteriophages
Specificity of bacteriophages
Very often only certain strains within a species are sensitive to a given bacteriophage. The bacterial host range is influenced by interactions between phage and its host. A phage recognizes and binds specifically to a host bacterium by receptor-binding proteins (RBPs) which are the first determinants of host specificity. Then, phage infectivity is also dependent on transport of DNA/RNA into the host cell and avoidance of bacteria defence mechanisms. Other factors such as structural changes of both phages and bacteria or presence of plasmids in a host cell can also play a role in a sensitivity to individual bacteriophages. Narrow host range, comparing to most antimicrobial drugs, may be seen as a limitation in a therapy, however, it is a guarantee of no interaction with a normal flora as phages target only a given pathogenic strain.
Phage Resistance and Implications for Therapy
For instance, they can transform into hardy spores, capable of surviving harsh conditions such as extreme temperatures and lack of nutrient. They may also eliminate genes responsible for antibiotic susceptibility or undergo mutations in receptors and other proteins targeted by antimicrobial agents and bacteriophages. It’s crucial to highlight that when bacteria develop resistance to bacteriophages, it’s often unfavourable for bacterial cell. This is because the receptors recognized by phages typically play a role in the bacteria’s ability to cause disease. Consequently, modifications to these receptors lead to a loss of virulence and can make the bacteria more vulnerable to other phages or antimicrobials. Furthermore, it’s worth noting that bacteria develop resistance to phages at a rate approximately ten times slower than they do for antibiotics. Additionally, becoming resistant to one type of phage doesn’t mean they’re immune to all phages. That’s why a combination of different phages, known as phage cocktails, is frequently employed to combat the same species of bacteria.
Maintaining Phage Effectiveness Over Time
Proper selection involves selecting and combining multiple phages to target a specific pathogenic bacterial strain or strains. Phage cocktails can be a powerful tool for phage therapy, biocontrol, or other applications. It is important to choose phages with diverse characteristics, such as different (but overlapping) host ranges, lytic mechanisms, and genetic backgrounds. This diversity can increase the effectiveness of the cocktail, as it’s not? likely that bacteria will develop resistance to multiple phages simultaneously. Some phages may work better together, enhancing their overall lytic activity that is why it is important to test the phages in combinations to assess their synergistic effects.
This video has been produced in collaboration with Phage EU.
Where Do We Operate
Sources
Maganha de Almeida Kumlien AC et al. (2021) Antimicrobial Resistance and Bacteriophages: An Overlooked Intersection in Water Disinfection, ICRA
Derek M Lin et al. (2017) Phage therapy: An alternative to antibiotics in the age of multi-drug resistance, New Mexico VA Health Care System
Laura M. Kasman and La Donna Porter (2024) Bacteriophages, StatPearls
Ana G. Abril et al. (2022) The Use of Bacteriophages in Biotechnology and Recent Insights into Proteomics, University of Santiago de Compostela, CSIC, Georgetown University, University of Sydney
Zuzanna Drulis-Kawa et al. (2015) Bacteriophages and Phage-Derived Proteins – Application Approaches, Institute of Genetics and Microbiology
Felipe Molina et al. (2022) Systematic analysis of putative phage-phage interactions on minimum-sized phage cocktails, Universidad de Extremadura, Instituto de Productos Lácteos de Asturias
Vimathi S. Gummalla et al. (2023) The Role of Temperate Phages in Bacterial Pathogenicity, USDA
Kaitlyn E.Kortright et al. (2019) Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria, Yale School of Medicine, Yale University
Majid Taati Moghadam et al. (2020) How Phages Overcome the Challenges of Drug Resistant Bacteria in Clinical Infections, Iran University of Medical Sciences
Proteon Pharmaceuticals Internal Resources
